
Eversense E3 CGM Approved for Two Sensors per Year: Your “Happily Ever(sense) After” - Taking Control Of Your Diabetes®

Germantown's Senseonics Announces FDA Approval of the World's First and Only Long-Term Implantable Glucose Monitoring System - The MoCo Show
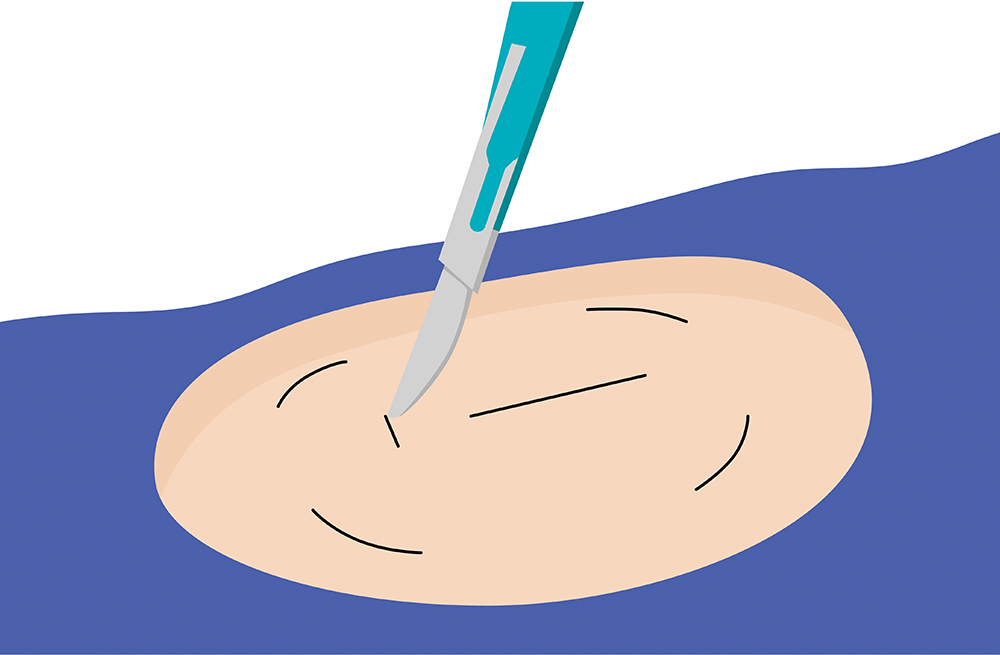
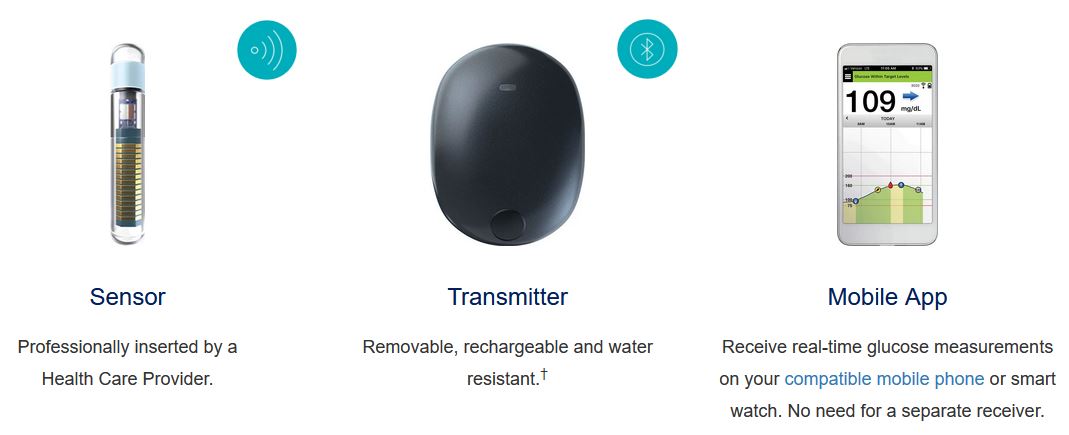
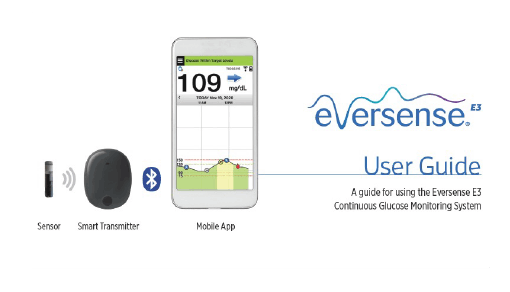
Break free from frequent and sometimes painful self-insertions weekly or bi-weekly. The Eversense sensor is carefully placed under the skin by a trained health care provider and lasts up to 6 months.
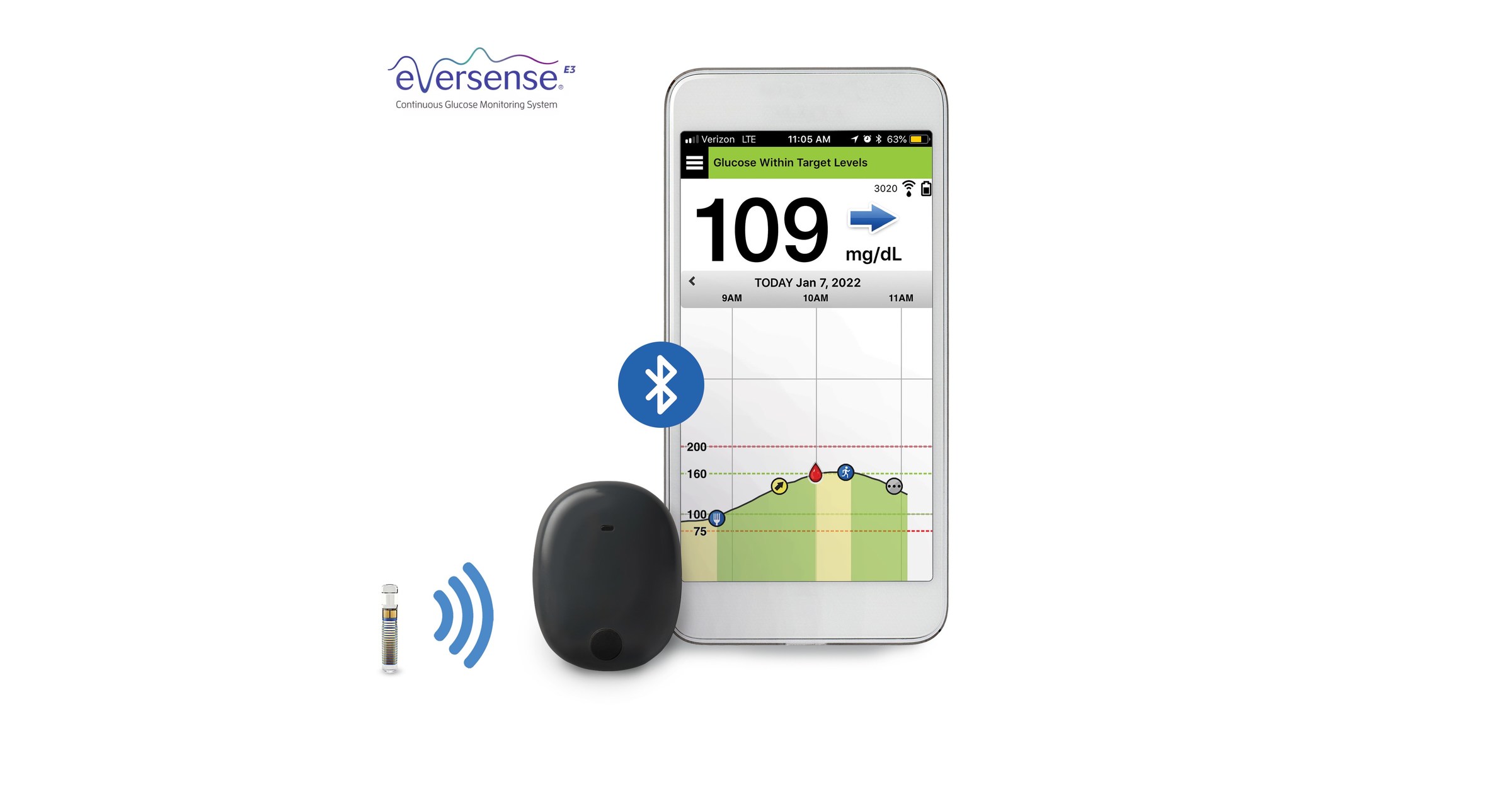
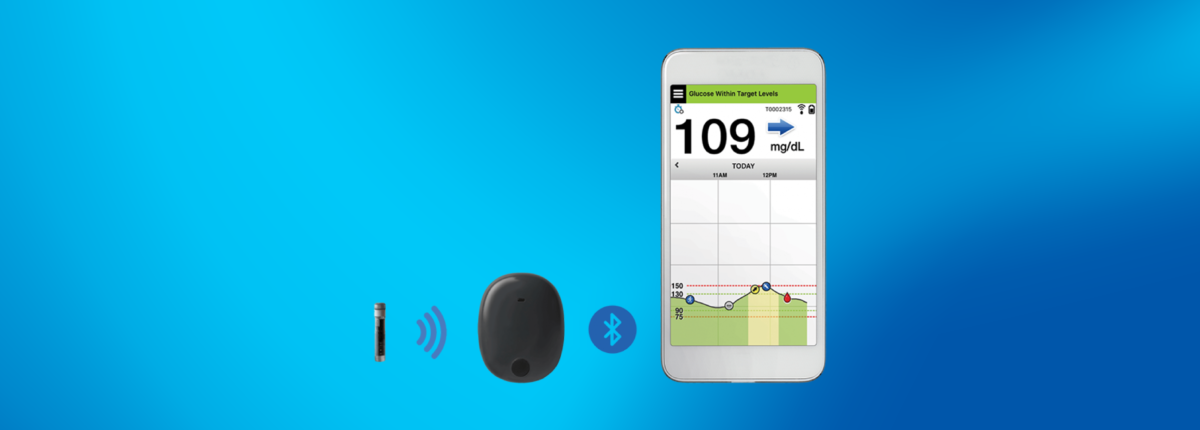

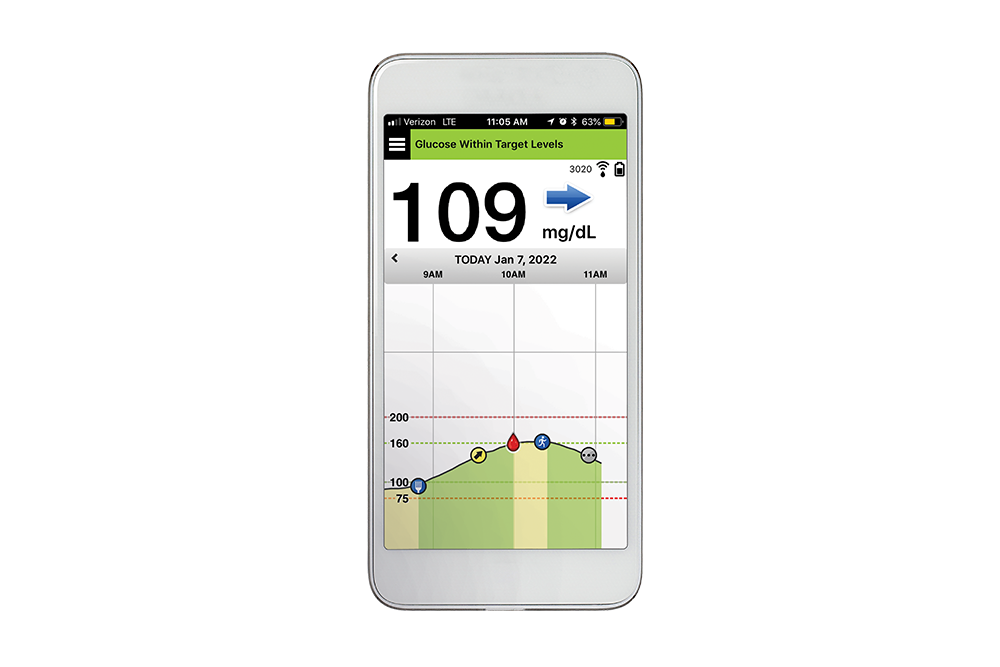











.jpg)
