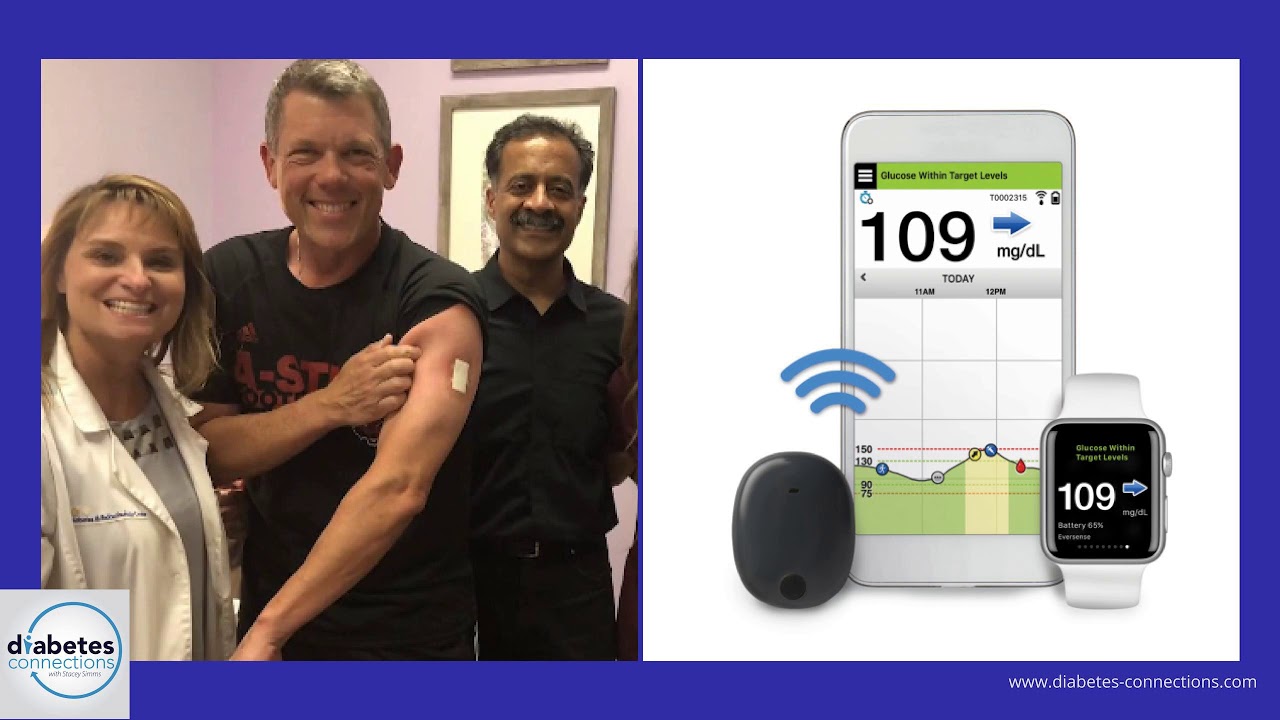
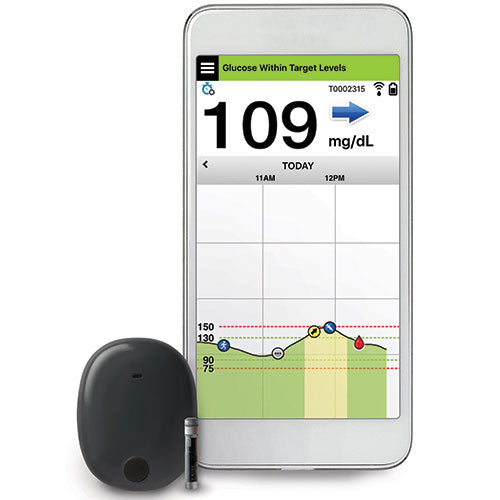
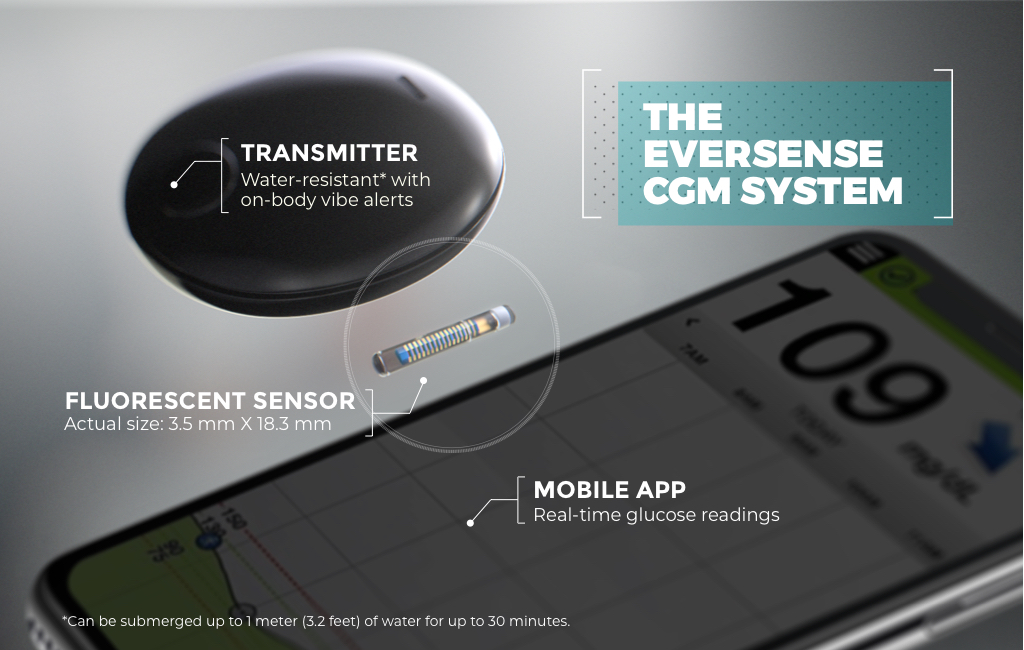
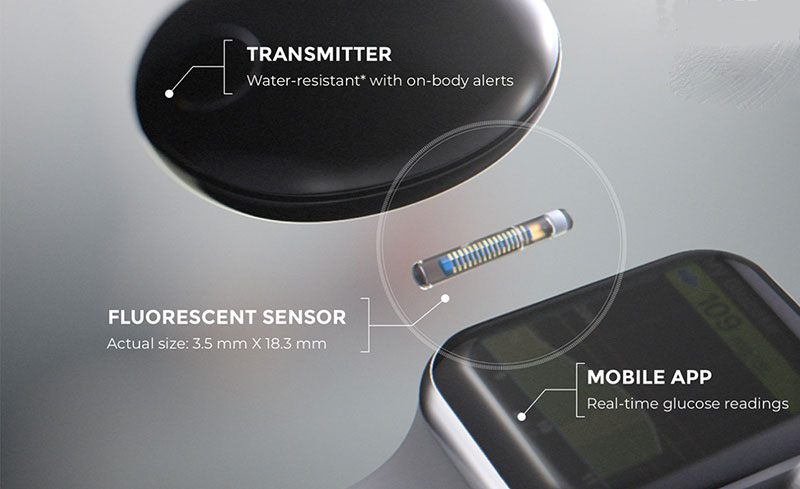
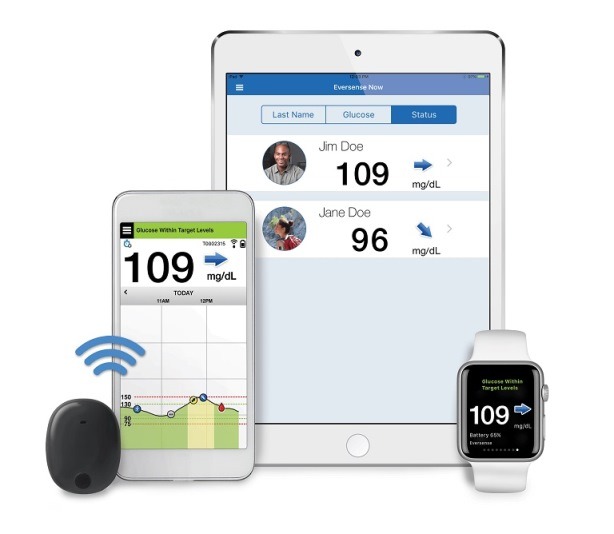
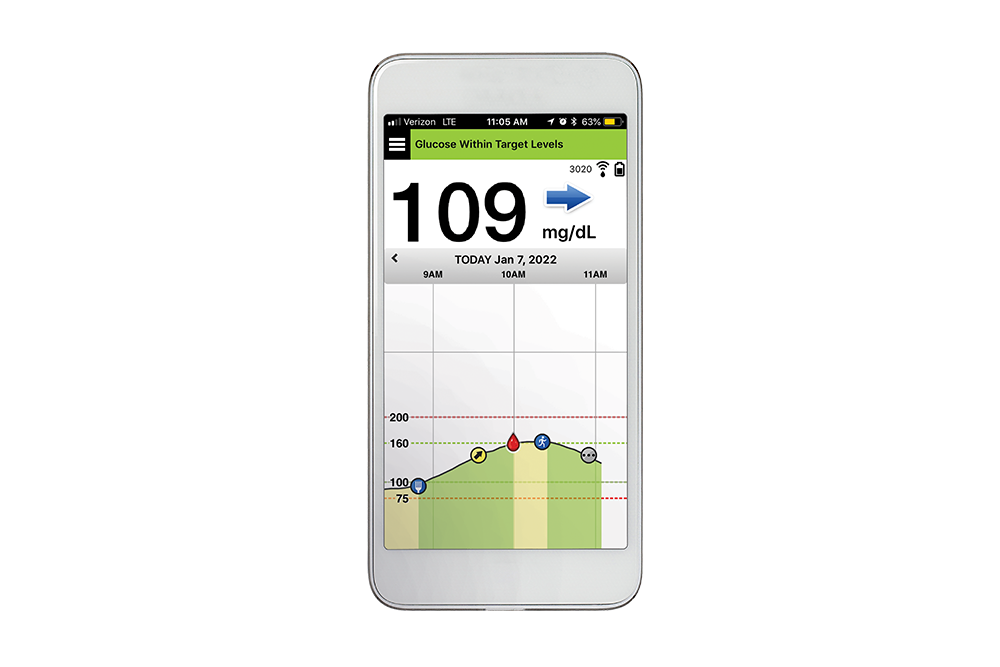
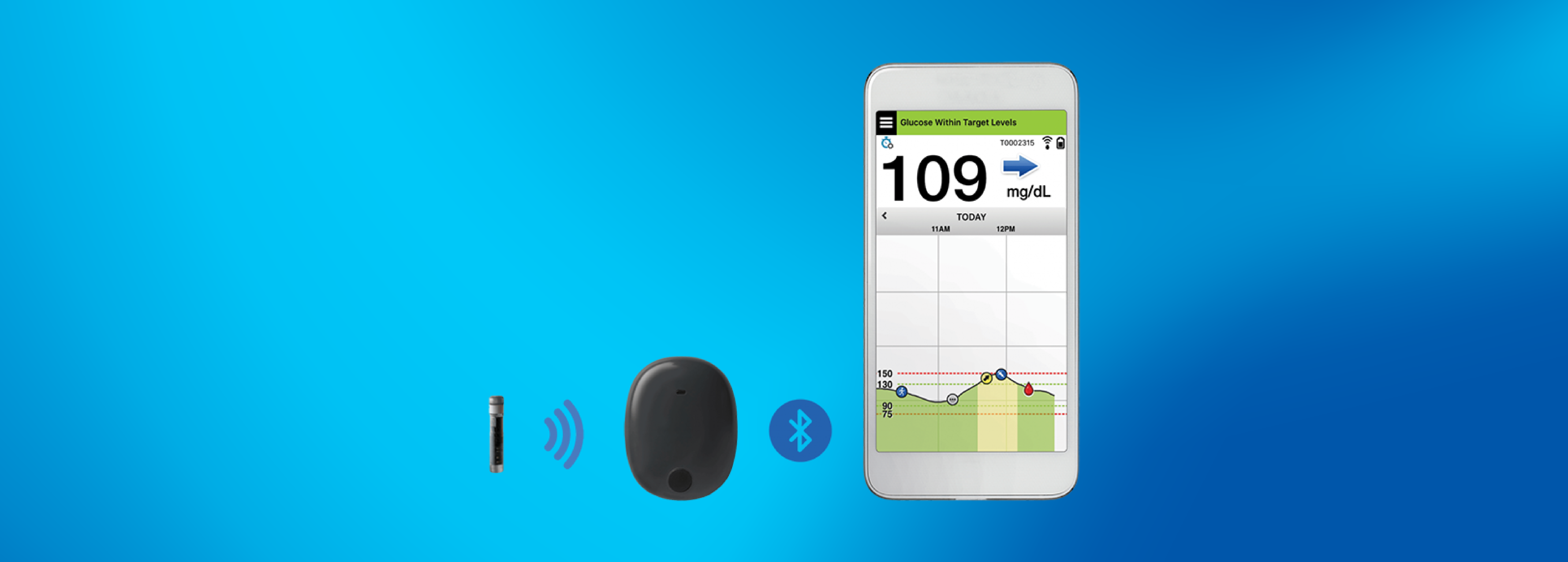
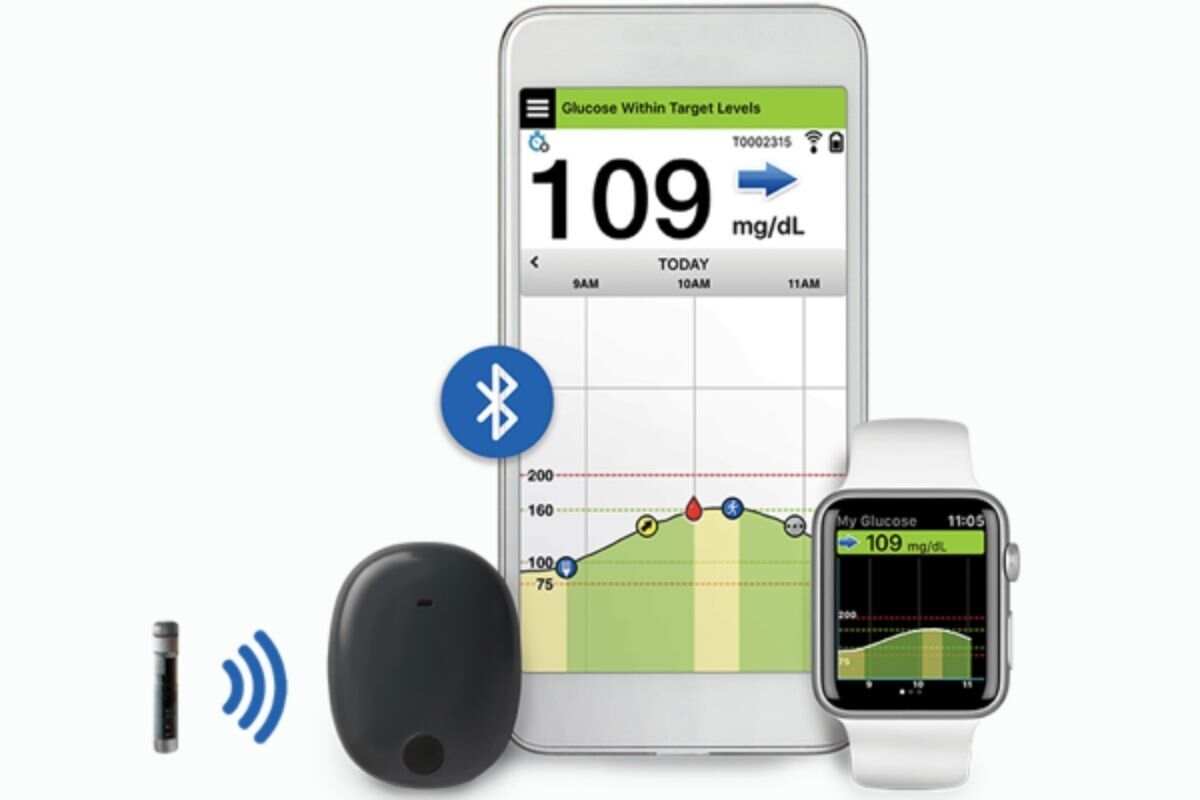
Beyond Type 1 - NOW APPROVED BY THE FDA - a new CGM is on the scene! The Eversense from Senseonics is the first implantable continuous glucose monitor. It lasts 90 days

Eversense E3 CGM Approved for Two Sensors per Year: Your “Happily Ever(sense) After” - Taking Control Of Your Diabetes®