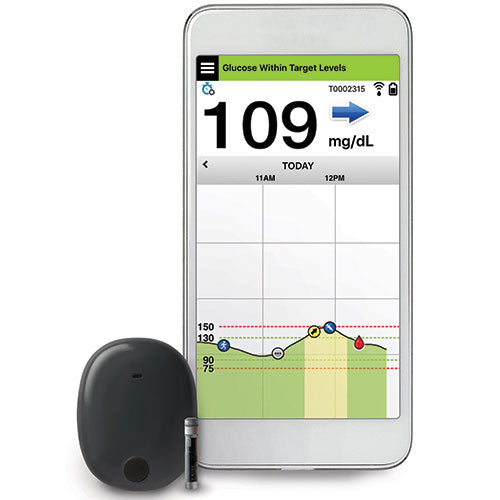
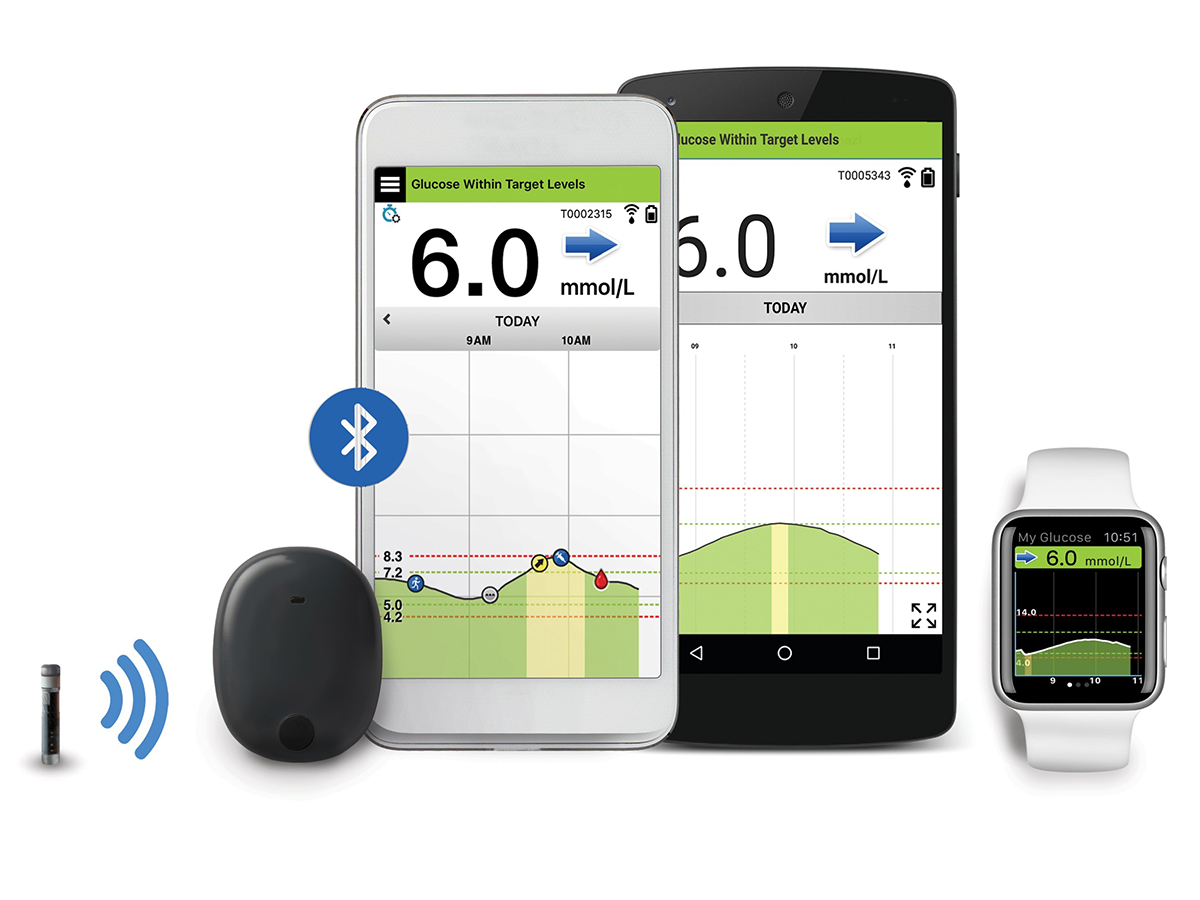
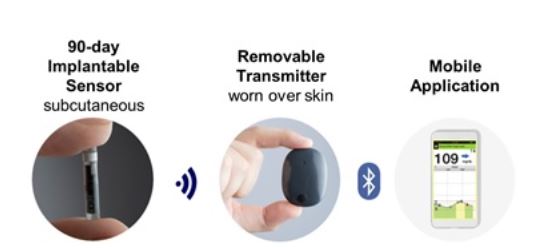
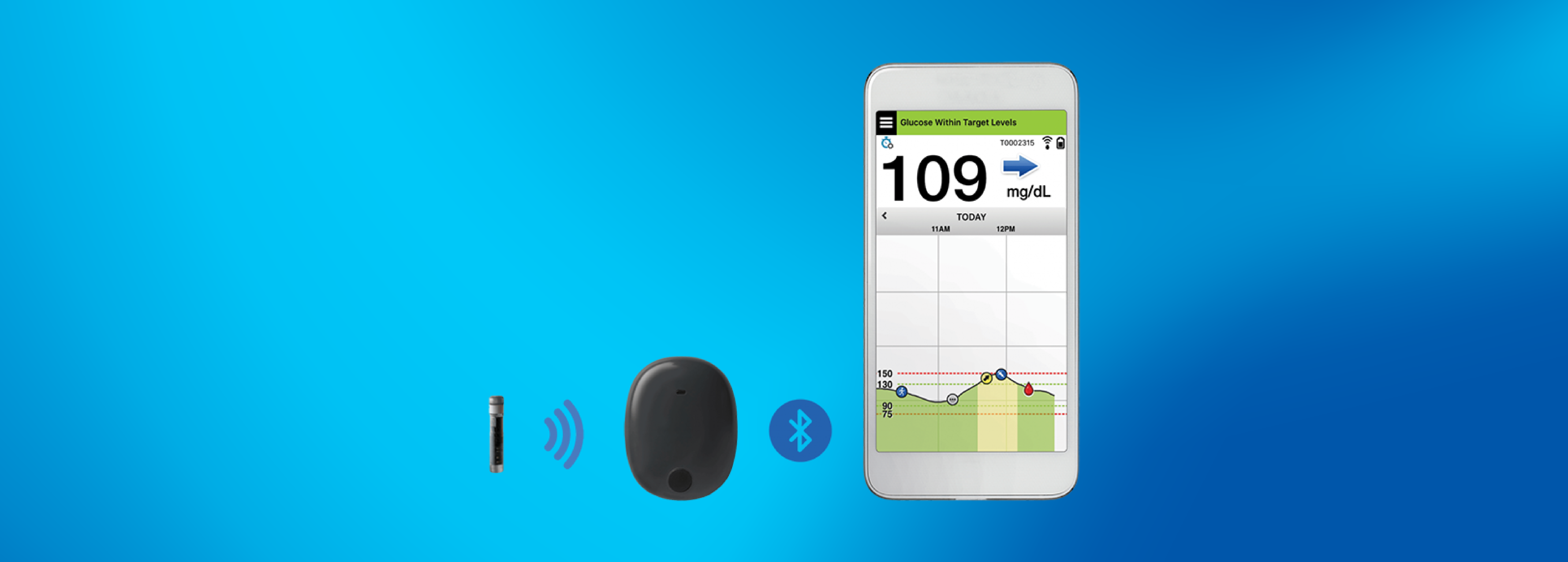
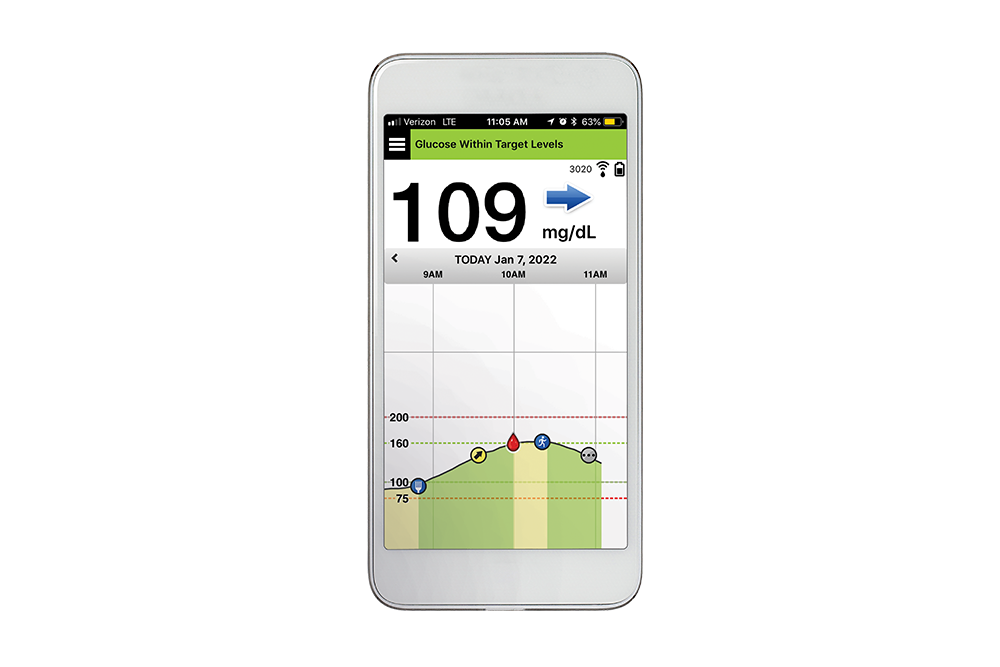
Implantable glucose sensor featuring IDT sensing technology awarded CE Mark - Medical Design and Outsourcing
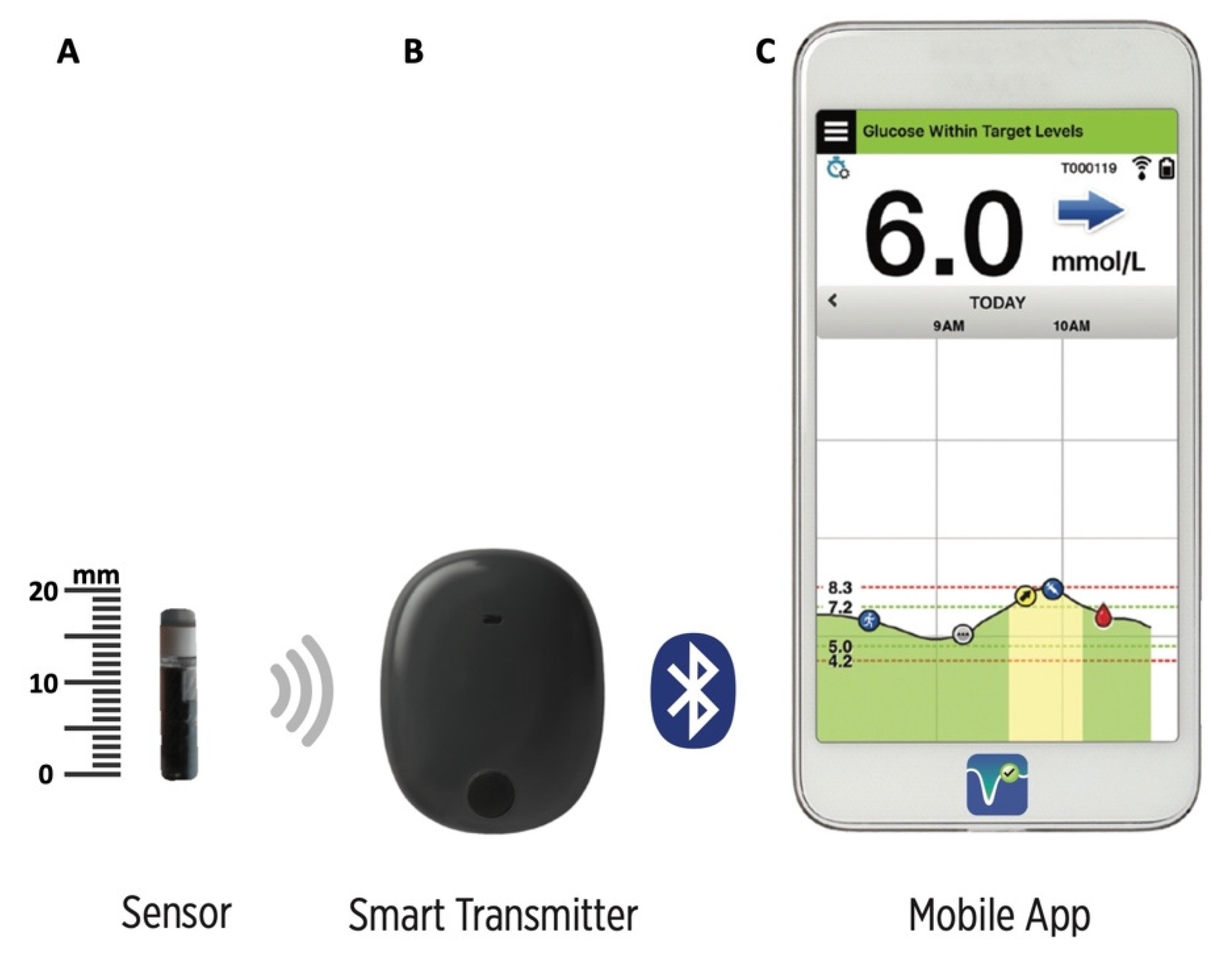
Animals | Free Full-Text | Clinical Use of a 180-Day Implantable Glucose Monitoring System in Dogs with Diabetes Mellitus: A Case Series