
Expanding Patient Access to Investigational Drugs: Single Patient Investigational New Drug and the “Right to Try” | JACC: Basic to Translational Science

Expanding Patient Access to Investigational Drugs: Single Patient Investigational New Drug and the “Right to Try” - ScienceDirect

Retrospective evaluation of single patient investigational new drug (IND) requests in pediatric oncology - Shulman - 2021 - Cancer Medicine - Wiley Online Library

PDF) Expanding Patient Access to Investigational Drugs: Single Patient Investigational New Drug and the “Right to Try”
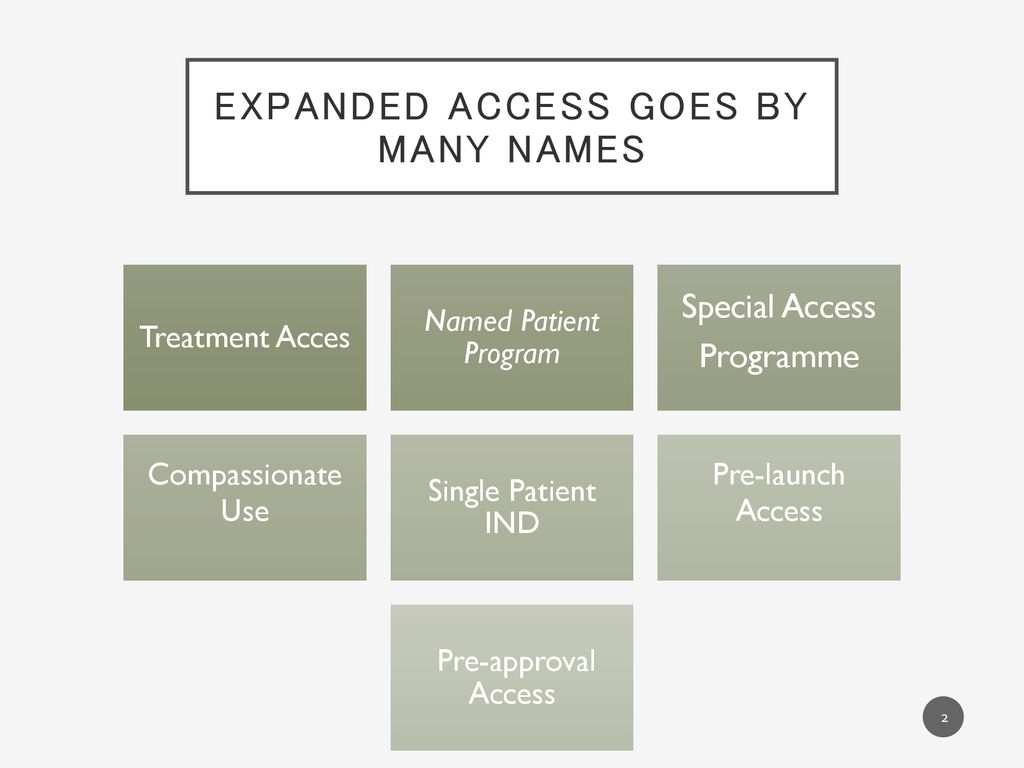
Intermediate-Size Patient Populations INDs: What Are They, When Should They Be Used, and Who May Apply for Them?” Richard Klein, Former Director, FDA. - ppt download




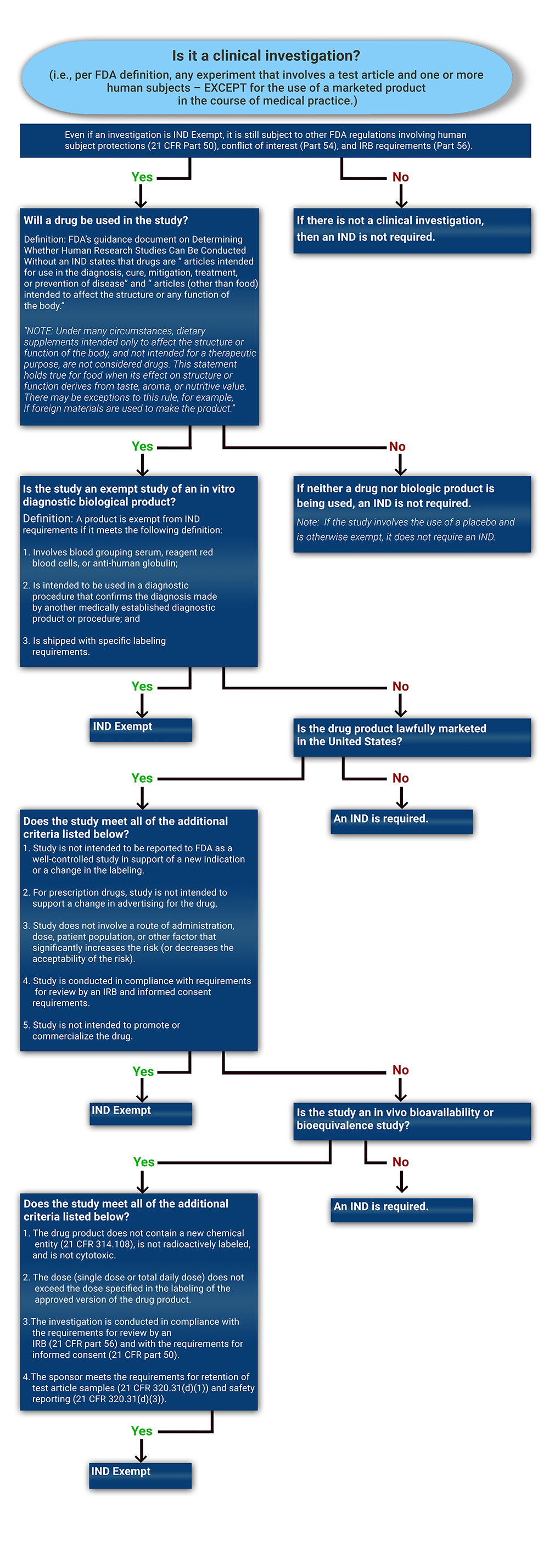



![INVESTIGATIONAL NEW DRUG [IND] APPLICATION SUBMISSION (1).pdf INVESTIGATIONAL NEW DRUG [IND] APPLICATION SUBMISSION (1).pdf](https://image.slidesharecdn.com/investigationalnewdrugindapplicationsubmission1-221128141339-adad00bd/85/investigational-new-drug-ind-application-submission-1pdf-27-320.jpg?cb=1669645214)






